In this tutorial, we will illustrate how to visualize and interpret Numbat output, using a triple-negative breast cancer dataset (TNBC1) from Gao et al. We will use pagoda2 for visualizing cells in low-dimensional expression space.
library(ggplot2)
library(numbat)
library(dplyr)
library(glue)
library(data.table)
library(ggtree)
library(stringr)
library(tidygraph)
library(patchwork)After a Numbat run, we can summarize output files into a Numbat object:
nb = Numbat$new(out_dir = './numbat_out')In this tutorial, let’s start the analysis with the pre-saved Numbat and pagoda2 objects.
nb = readRDS(url('http://pklab.org/teng/data/nb_TNBC1.rds'))
pagoda = readRDS(url('http://pklab.org/teng/data/con_TNBC1.rds'))Copy number landscape and single-cell phylogeny
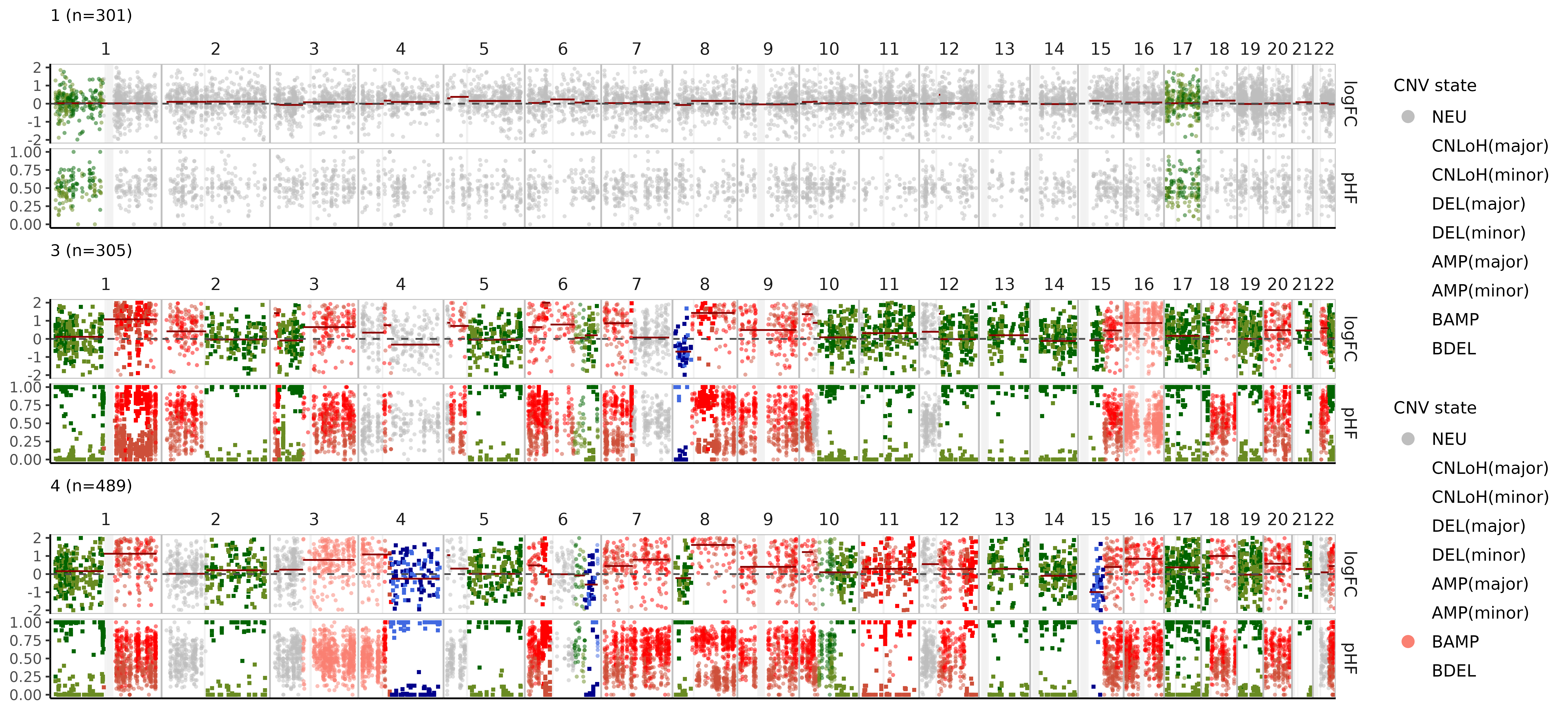
As an overview, we can visualize the CNV calls in single-cells and their evolutionary relationships in an integrated plot panel:
mypal = c('1' = 'gray', '2' = "#377EB8", '3' = "#4DAF4A", '4' = "#984EA3")
nb$plot_phylo_heatmap(
clone_bar = TRUE,
p_min = 0.9,
pal_clone = mypal
)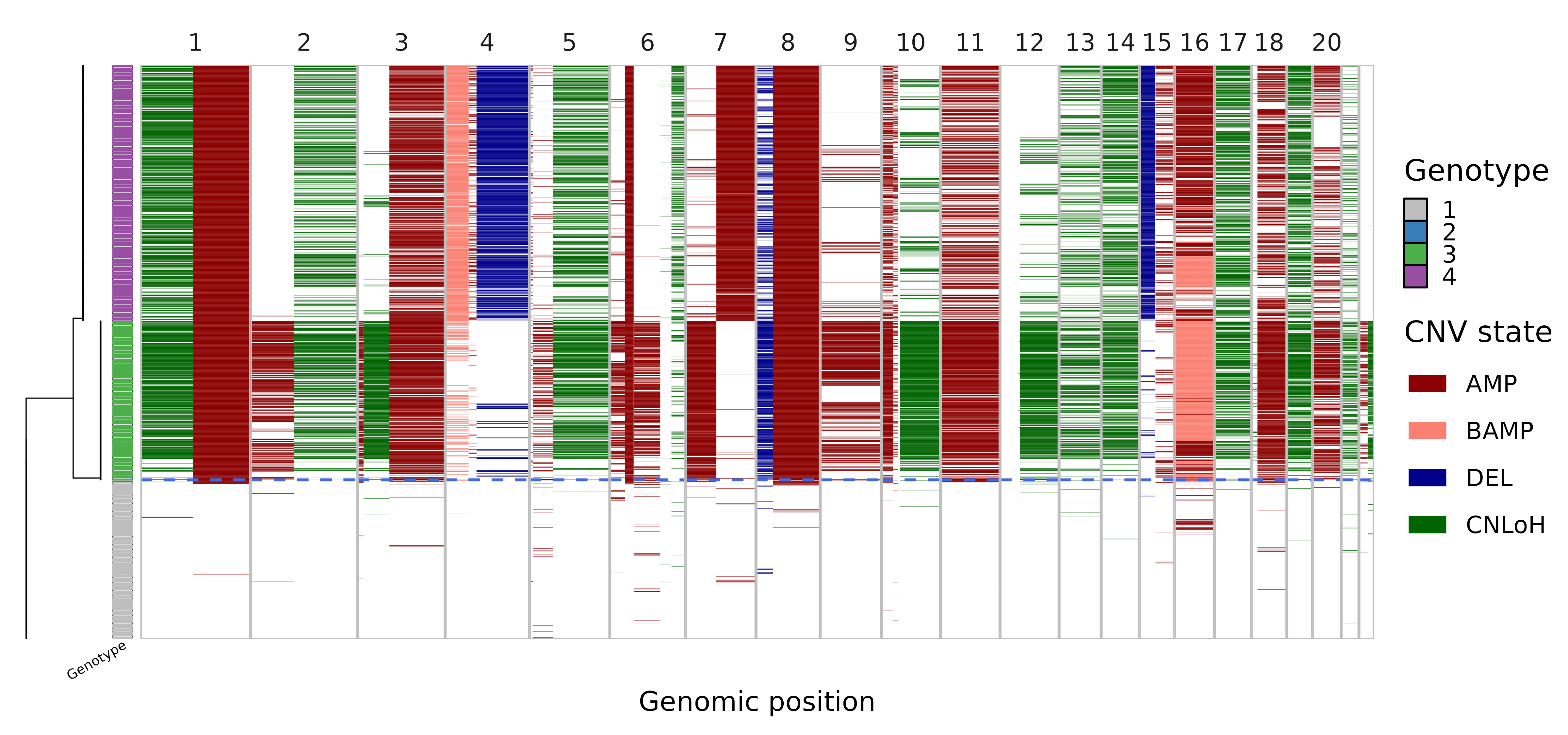 In this visualization, the single-cell phylogeny (left) is juxtaposed
with a heatmap of single-cell CNV calls (right). The CNV calls are
colored by the type of alterations (AMP, amplification, BAMP, balanced
amplification, DEL, deletion, CNLoH, copy-neutral loss of
heterozygosity). The colorbar in-between differentiates the distinct
genetic populations (genotype). The dashed blue line separates the
predicted tumor versus normal cells. This tells us that the dataset
mainly consists of three cell populations, a normal population (gray)
and two tumor subclones (green and purple).
In this visualization, the single-cell phylogeny (left) is juxtaposed
with a heatmap of single-cell CNV calls (right). The CNV calls are
colored by the type of alterations (AMP, amplification, BAMP, balanced
amplification, DEL, deletion, CNLoH, copy-neutral loss of
heterozygosity). The colorbar in-between differentiates the distinct
genetic populations (genotype). The dashed blue line separates the
predicted tumor versus normal cells. This tells us that the dataset
mainly consists of three cell populations, a normal population (gray)
and two tumor subclones (green and purple).
Refine subclones on the phylogeny
Note that the number of subclones determined by the initial run
parameters in run_numbat can be re-adjusted using
nb$cutree():
plots = lapply(
1:4,
function(k) {
nb$cutree(n_cut = k)
nb$plot_phylo_heatmap() + ggtitle(paste0('n_cut=', k))
}
)
wrap_plots(plots)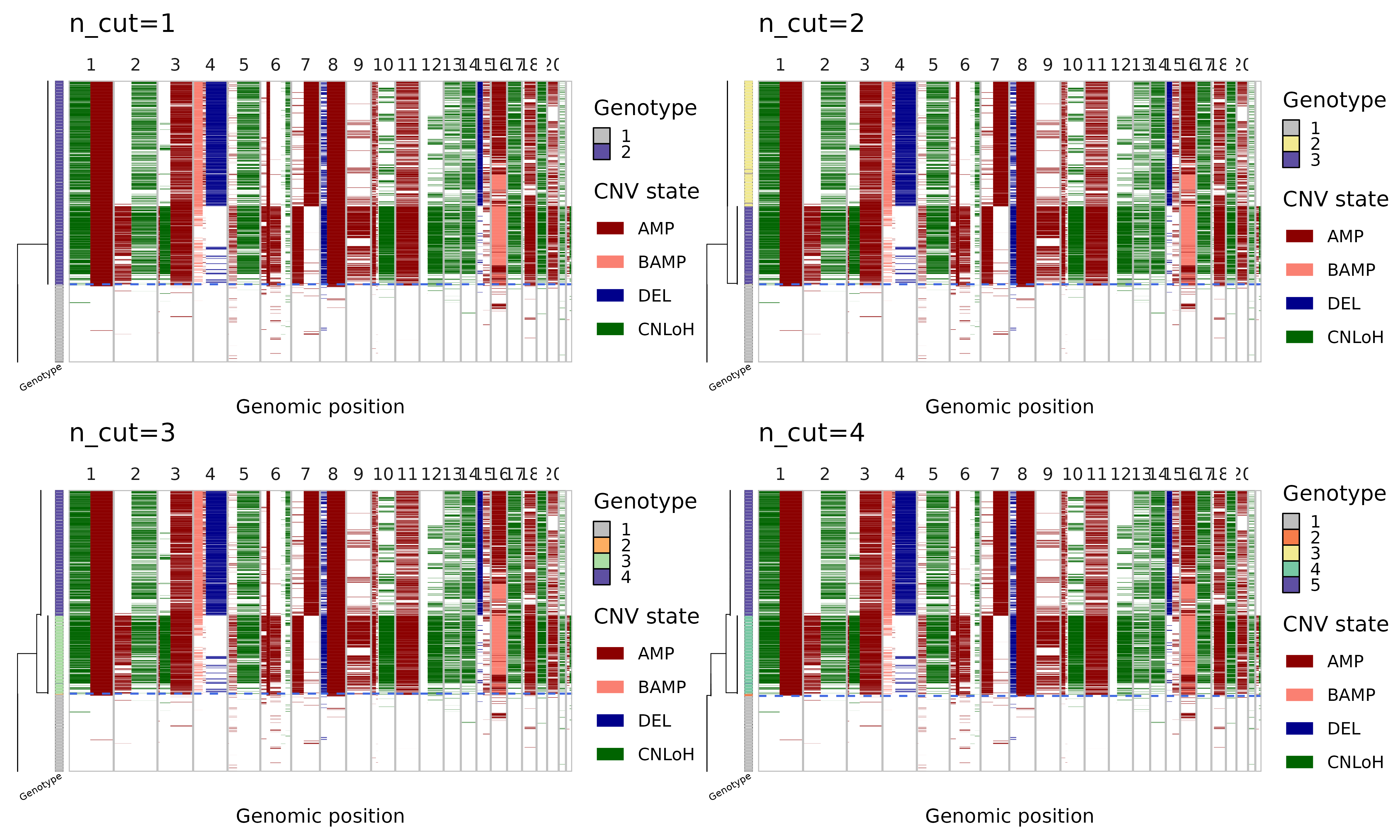
In cutree one can either specify n_cut or
max_cost, which work similarly as k and
h in stats::cutree. Note that n
cuts should result in n+1 clones (the top-level normal
diploid clone is always included). First cut should separate out the
tumor cells as a single clone, second cut gives two tumor subclones, and
so on. Alternatively, one can specify a max_cost, which is
the maximum likelihood cost threshold with which to reduce the phylogeny
(higher max_cost leads to fewer clones).
# restore to original number of cuts
nb$cutree(n_cut = 3)After refining subclones in the phylogeny, you can recreate the clone pseduobulk profiles and visualize them (see issue #220).
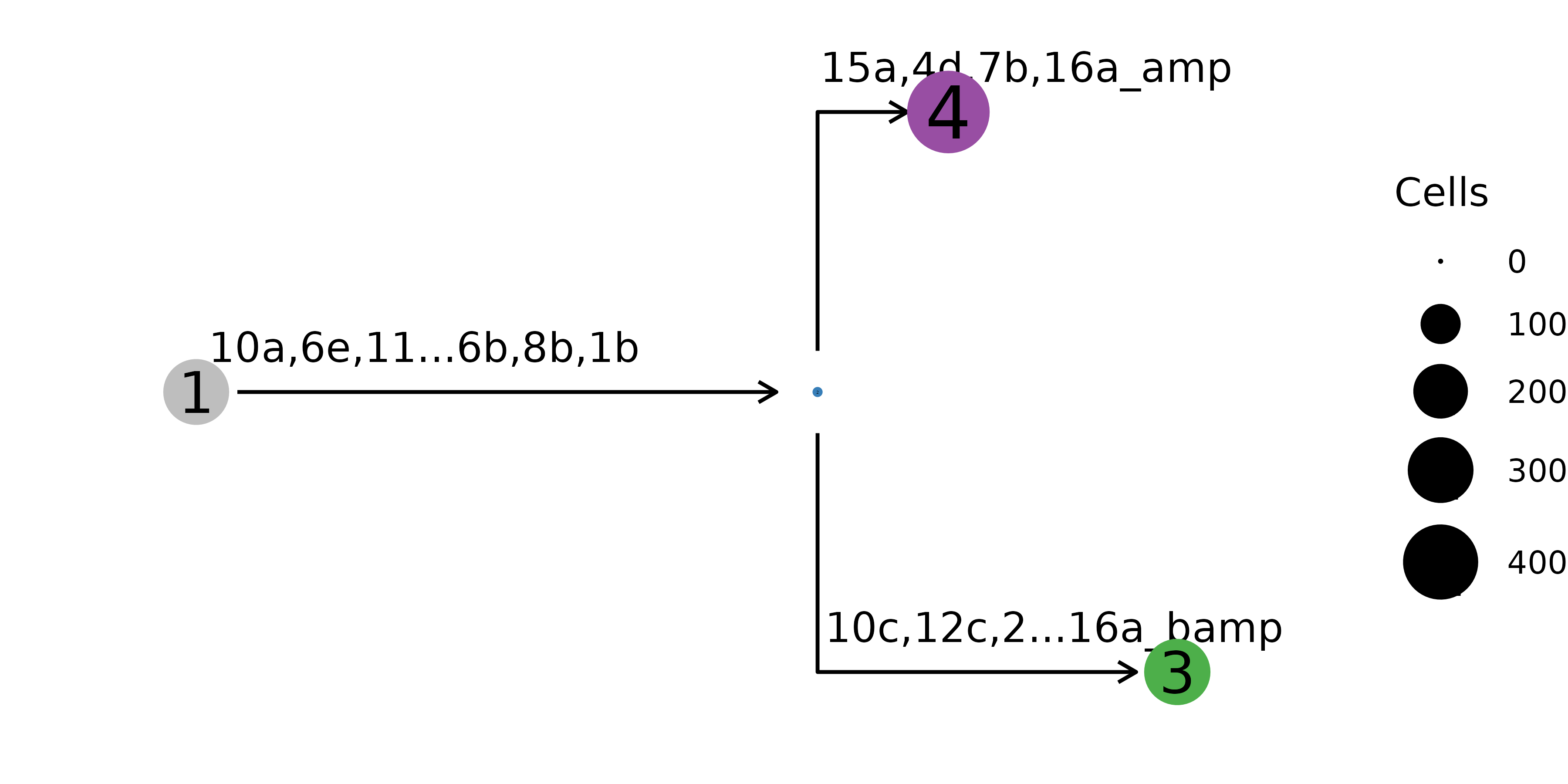
Bulk CNV profiles
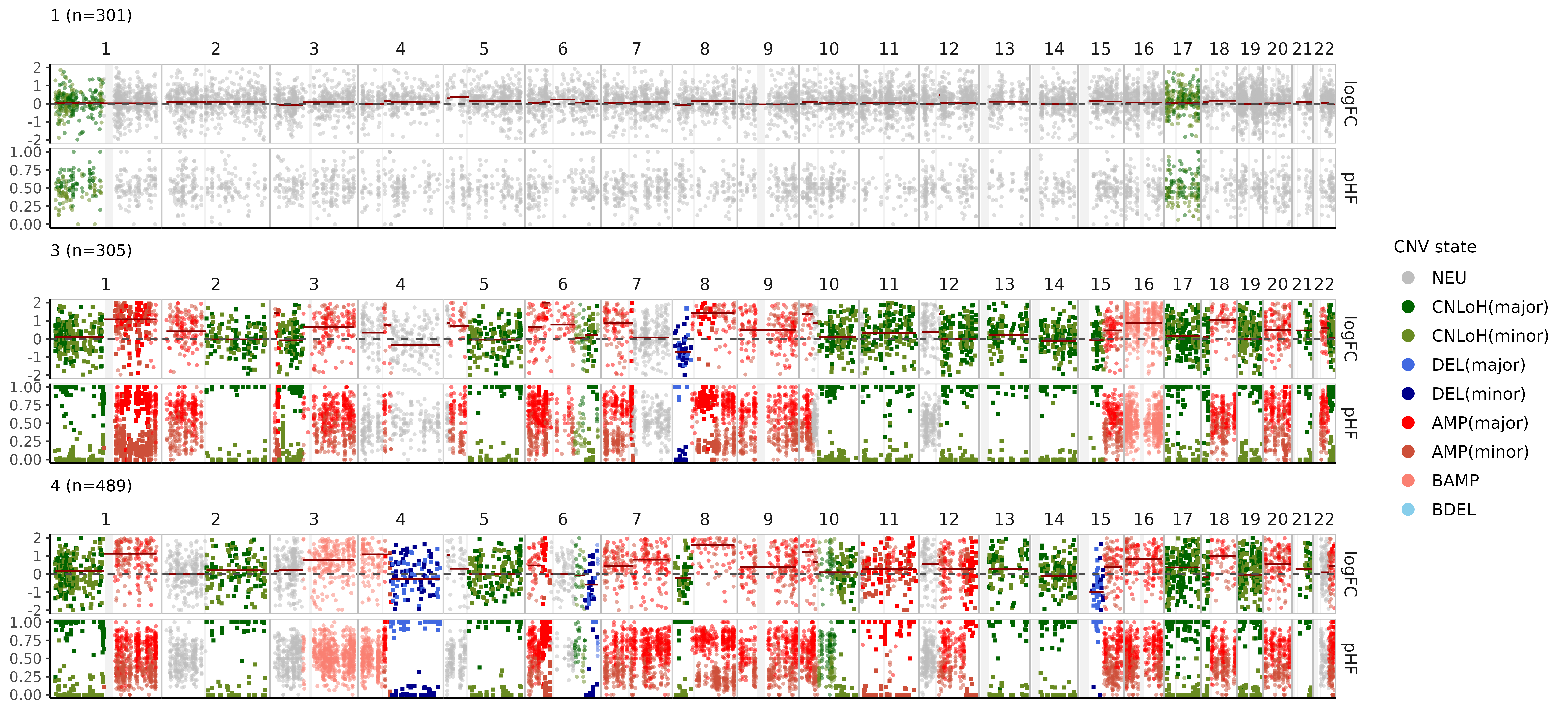
We can also visualize these CNV events in pseudobulks where the data is more rich, aggregating cells by clone:
nb$bulk_clones %>%
filter(n_cells > 50) %>%
plot_bulks(
min_LLR = 10, # filtering CNVs by evidence
legend = TRUE
)
Single-cell CNV calls
Numbat probabilistically evaluates the presence/absence of CNVs in
single cells. The cell-level CNV posteriors are stored in the
nb$joint_post dataframe:
## cell CHROM seg cnv_state p_cnv p_cnv_x
## <char> <fctr> <char> <char> <num> <num>
## 1: TNBC1_AAACCTGCACCTTGTC-1 1 1b amp 0.9999999997 1.000000000
## 2: TNBC1_AAACCTGCACCTTGTC-1 2 2a amp 0.0002288397 0.001172894
## 3: TNBC1_AAACCTGCACCTTGTC-1 3 3a amp 0.0522751360 0.052273344
## 4: TNBC1_AAACCTGCACCTTGTC-1 3 3c amp 0.9147696618 0.982278728
## 5: TNBC1_AAACCTGCACCTTGTC-1 4 4b amp 0.8999658266 0.845619643
## 6: TNBC1_AAACCTGCACCTTGTC-1 5 5a amp 0.8070027725 0.778074328
## p_cnv_y
## <num>
## 1: 0.03844235
## 2: 0.09821435
## 3: 0.50000000
## 4: 0.10246769
## 5: 0.61862288
## 6: 0.53344303which contains cell-level information on specific CNV segments
(seg), their alteration type (cnv_state), the
joint posterior probability of the CNV (p_cnv), the
expression-based posterior (p_cnv_x), and the allele-based
posterior (p_cnv_y). We can visualize the event-specific
posteriors in a expression-based tSNE embedding:
plist = list()
muts = c('1a', '3b', '22b')
cnv_type = nb$joint_post %>% distinct(seg, cnv_state) %>% {setNames(.$cnv_state, .$seg)}
for (mut in muts) {
plist[[mut]] = pagoda$plotEmbedding(
alpha=0.8,
size=1,
plot.na = F,
colors = nb$joint_post %>%
filter(seg == mut) %>%
{setNames(.$p_cnv, .$cell)},
show.legend = T,
mark.groups = F,
plot.theme = theme_bw(),
title = paste0(mut, '(', cnv_type[muts], ')')
) +
scale_color_gradient2(low = 'royalblue', mid = 'white', high = 'red3', midpoint = 0.5, limits = c(0,1), name = 'Posterior')
}
wrap_plots(plist, guides = 'collect')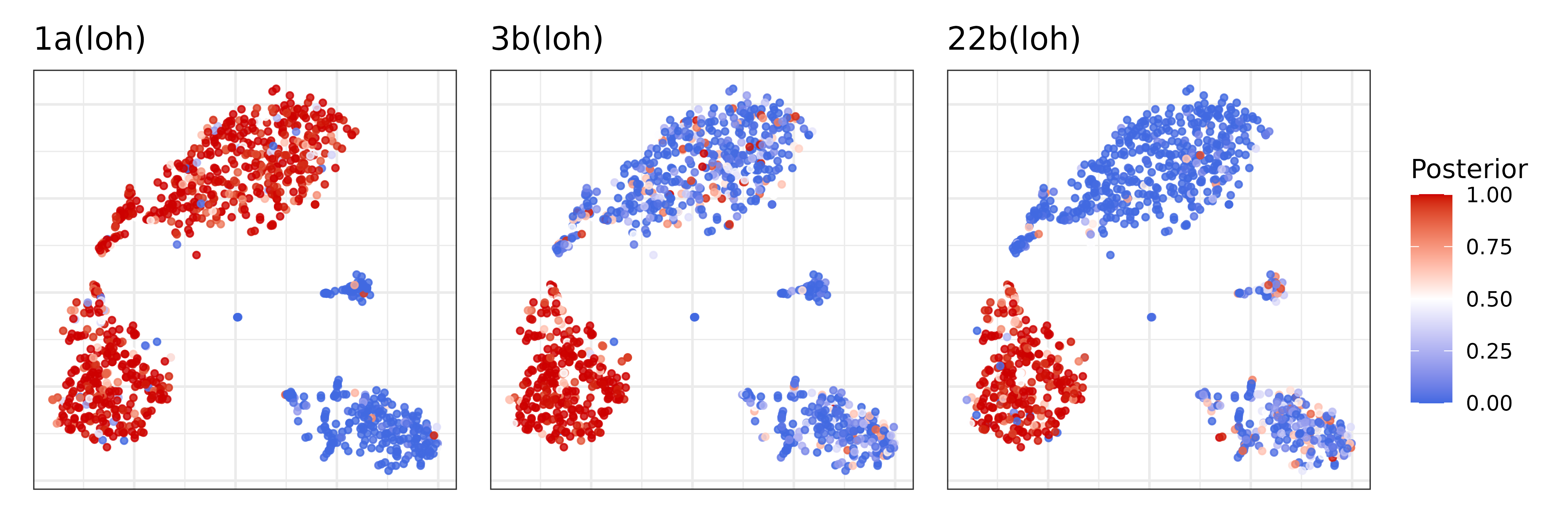
Clonal assignments
Numbat aggregates signals across subclone-specific CNVs to
probabilistically assign cells to subclones. The information regarding
clonal assignments are contained in the nb$clone_post
dataframe.
## cell clone_opt p_1 p_2 p_3
## 1 TNBC1_AAACCTGCACCTTGTC-1 4 1.162495e-72 2.105425e-20 3.478936e-29
## 2 TNBC1_AAACGGGAGTCCTCCT-1 1 1.000000e+00 5.814741e-51 5.920463e-95
## 3 TNBC1_AAACGGGTCCAGAGGA-1 4 2.434561e-100 2.175022e-14 2.085718e-30
## 4 TNBC1_AAAGATGCAGTTTACG-1 3 2.020088e-23 8.164920e-04 9.913458e-01
## 5 TNBC1_AAAGCAACAGGAATGC-1 4 9.546324e-64 4.989037e-11 1.657378e-31
## 6 TNBC1_AAAGCAATCGGAATCT-1 3 1.516589e-99 8.466806e-33 1.000000e+00
## p_4
## 1 1.000000e+00
## 2 8.821034e-66
## 3 1.000000e+00
## 4 7.837745e-03
## 5 1.000000e+00
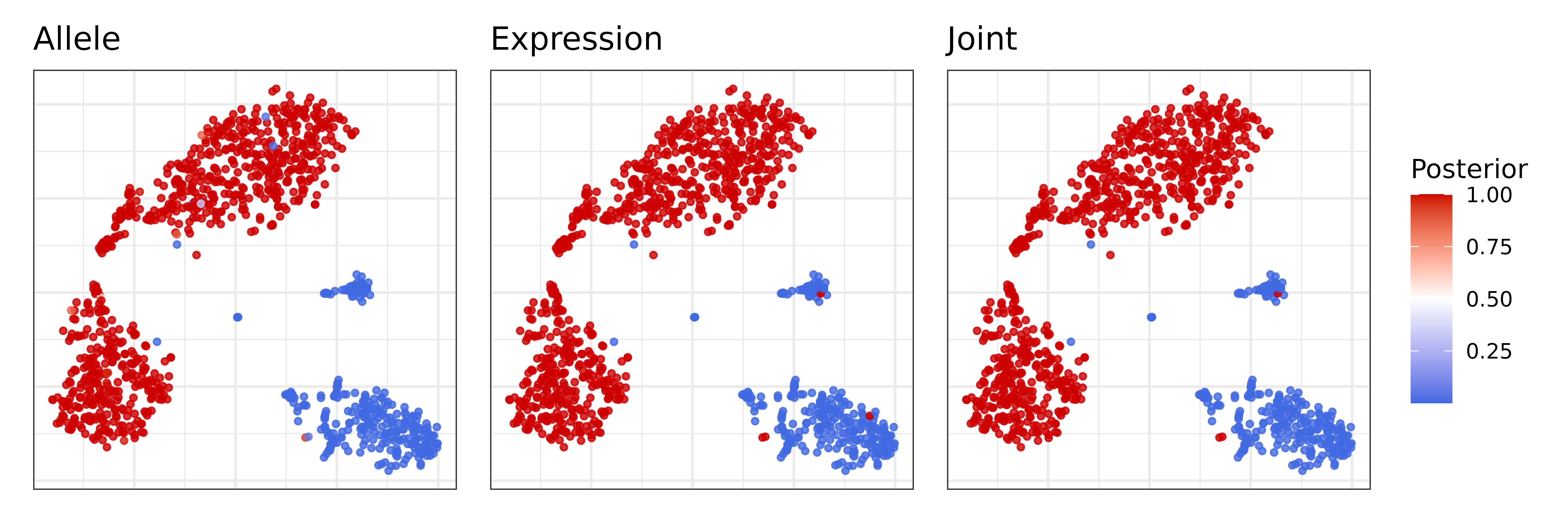
## 6 2.457592e-29Here clone_opt denotes the maximum likelihood assignment
of a cell to a specific clone. p_{1..4} are the detailed
breakdown of the posterior probability that the cell belongs to each
clone, respectively. Let’s visualize the clonal decomposition in a tSNE
embedding. Note that clone 1 is always the normal cells.
pagoda$plotEmbedding(
alpha=0.8,
size=1,
groups = nb$clone_post %>%
{setNames(.$clone_opt, .$cell)},
plot.na = F,
plot.theme = theme_bw(),
title = 'Genotypes',
pal = mypal
)
Tumor versus normal probability
Combining evidence from all CNVs, Numbat derives an aneuploidy probability for each cell to distinguish tumor versus normal cells. We can visualize the posterior aneuploidy probability based on expression evidence only, allele evidence only, and jointly:
p_joint = pagoda$plotEmbedding(
alpha=0.8,
size=1,
colors = nb$clone_post %>%
{setNames(.$p_cnv, .$cell)},
plot.na = F,
plot.theme = theme_bw(),
title = 'Joint',
) +
scale_color_gradient2(low = 'royalblue', mid = 'white', high = 'red3', midpoint = 0.5, name = 'Posterior')
p_allele = pagoda$plotEmbedding(
alpha=0.8,
size=1,
colors = nb$clone_post %>%
{setNames(.$p_cnv_x, .$cell)},
plot.na = F,
plot.theme = theme_bw(),
title = 'Expression',
) +
scale_color_gradient2(low = 'royalblue', mid = 'white', high = 'red3', midpoint = 0.5, name = 'Posterior')
p_expr = pagoda$plotEmbedding(
alpha=0.8,
size=1,
colors = nb$clone_post %>%
{setNames(.$p_cnv_y, .$cell)},
plot.na = F,
show.legend = T,
plot.theme = theme_bw(),
title = 'Allele',
) +
scale_color_gradient2(low = 'royalblue', mid = 'white', high = 'red3', midpoint = 0.5, name = 'Posterior')
(p_expr | p_allele | p_joint) + plot_layout(guides = 'collect')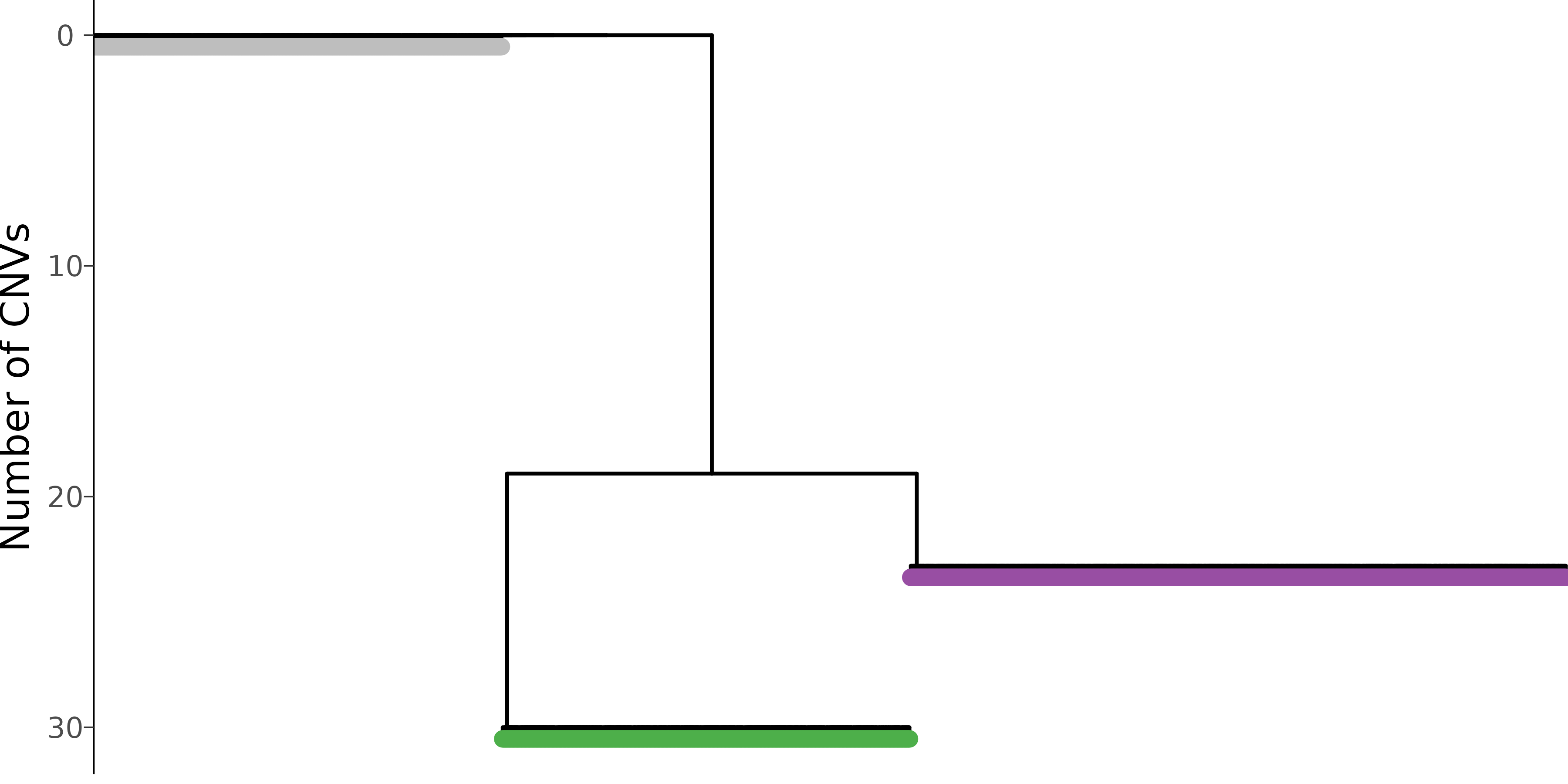 Both expression and allele signal clearly separate the tumor and normal
cells.
Both expression and allele signal clearly separate the tumor and normal
cells.
Tumor phylogeny
Let’s take a closer look at the inferred single cell phylogeny and where the mutations occurred.
nb$plot_sc_tree(
label_size = 3,
branch_width = 0.5,
tip_length = 0.5,
pal_clone = mypal,
tip = TRUE
)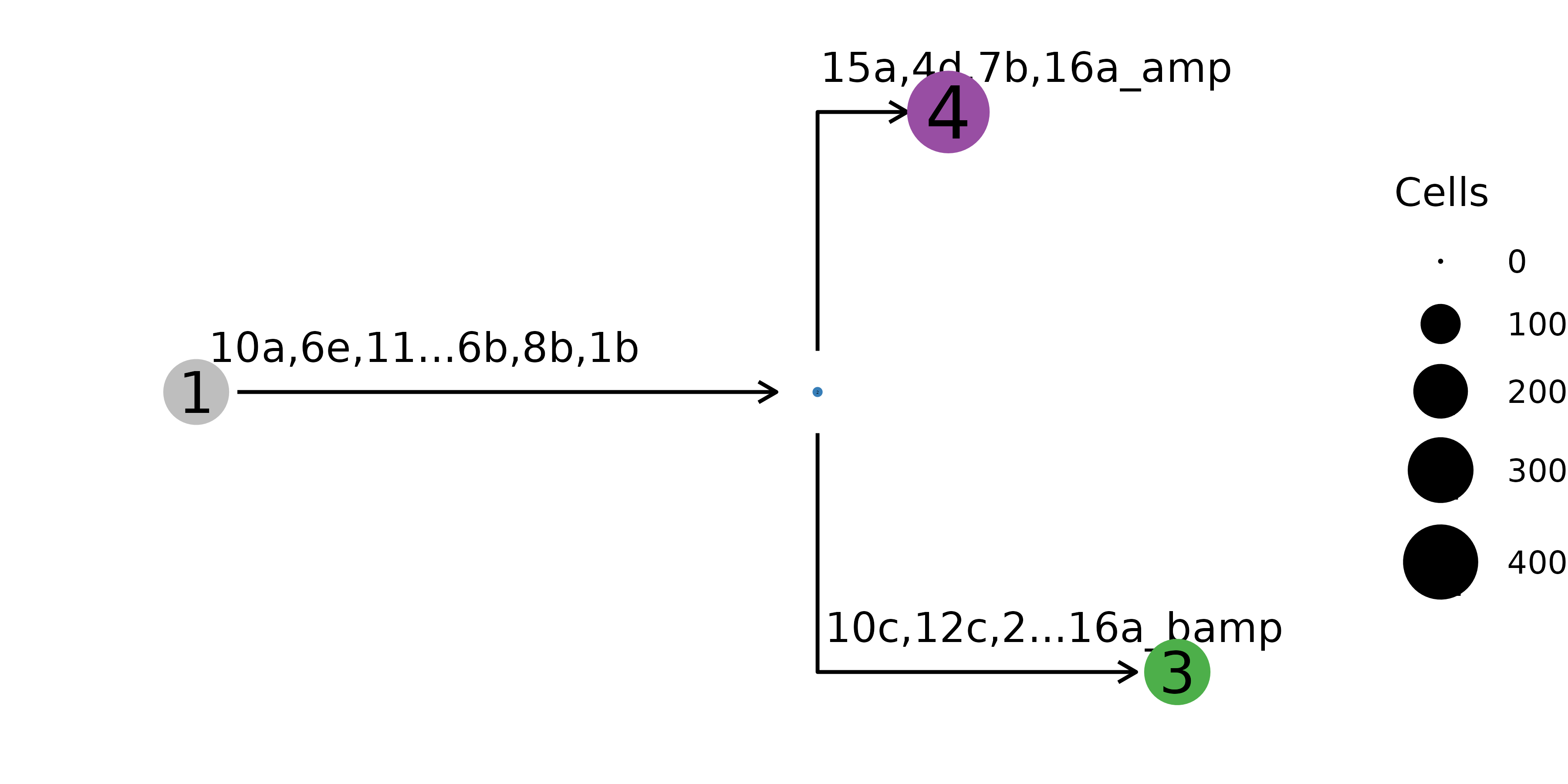 The mutational history can also be represented on the clone level, where
cells with the same genotype are aggregated into one node.
The mutational history can also be represented on the clone level, where
cells with the same genotype are aggregated into one node.
nb$plot_mut_history(pal = mypal)